OncoOVARIAN Dx – Ovarian Cancer Diagnosis Test
Overview
OncoOVARIAN Dx is an innovative, non-invasive, accurate, and cost-effective already validated test to help in Ovarian Cancer diagnosis as well as confirmatory diagnostic ―as an adjunct to suspicious image procedures findings, in order to reduce the number of unnecessary tissue biopsies that patients have to undergo―, with potentially uses for screening, prognosis and recurrence monitoring.
OncoOVARIAN Dx is based on a score calculation that it is obtained from several Biomarkers of the patient (mainly Tumor Markers but also patient’s clinical information).

Click here to download the brochure in PDF format.
Tumor Markers
Tumor Markers are parameters released by tumor cells, which enter the bloodstream or other biological fluids and are useful for the diagnosis, prognosis and treatment monitoring.
Most Tumor Markers are not specific to any type of cancer and the differences between benign and malignant diseases are quantitative (for example, patients with epithelial tumors tend to have significantly higher levels of these Tumor Markers than patients without malignancy).
There are now more than 20 well known parameters that are widely regarded as Tumor Markers such as PSA ―related to Prostate Cancer―, CA 15.3 ―related to Breast Cancer―, CA 125 and HE4 ―both related to Ovarian Cancer―, CEA and CA 19.9 ―both related to different gastrointestinal cancers (Colorectal, Gastric and Pancreatic Cancer)―, or NSE and ProGRP ―both related to in Lung Cancer―.
However, there are a variety of factors that can affect the accuracy of Tumor Markers by increasing its levels without malignancy presence. The main reason are benign diseases, among others, such as technical interferences.
In this sense, the Spanish Society of Clinical Biochemistry and Molecular Pathology, Cancer Biomarker Commission established the Barcelona Criteria, 4 criteria that help to correctly distinguish and value Tumor Markers results and reduce False Positives (FP):
- Tumor Markers Serum concentrations.
- Discard benign pathology by the exclusion of main source False Positive results.
- Follow-up if Tumor Markers moderate results (Grey Zone/Undetermined).
- Technical interference.
Statistical measurements in diagnostic tests
Unfortunately, the use of Tumor Markers in routine presents also other problems such as low Sensitivity in early stages, or nonexistence of any specific Tumor Marker for each malignant tumor. However, the combination of 2 or more Tumor Markers has a better outcome, especially in advanced stages.
In this regard, the combination of several Tumor Markers ―as well as the inclusion of patient history information in the equations―, using complex algorithms with multiple variables, results in higher Sensitivity and Specificity: that is what we have christened Multi-Biomarker Disease Activity Algorithm (MBDAA).
The Sensitivity of a diagnostic test is the percentage of actual positives that are correctly identified, and Specificity is the proportion of true negatives that are correctly classified. Both variables are closely linked together and give an idea of the accuracy of a test.
A test that correctly identifies all true positive as positive, but has many false negatives would have a Sensitivity of 100%, but low Specificity. For example, Sensitivity measures the number of cancerous tumors that are correctly identified as cancerous, whereas Specificity measures how many benign tumors are correctly identified as benign. A high Sensitivity means fewer cancers diagnosed as benign and high Specificity means fewer benign tumors diagnosed as cancerous.
Besides, the positive predictive value (PPV) is the number of true positives correctly identified on total real positive. A test with many false positives will have a low VPP. Moreover, the negative predictive value (NPV) is the number of true negatives correctly identified on the total actual negative. A high NPV value means that very few true positives were incorrectly identified as negative.
All these different values can be plotted together in a graphic that it is known as Receiving Operator Curve (ROC), where better results are displayed with curves that tends to come near to the upper left corner of the image (where 100% Sensitivity and 100% Specificity are reached).
Receiving Operator Curves (ROC)
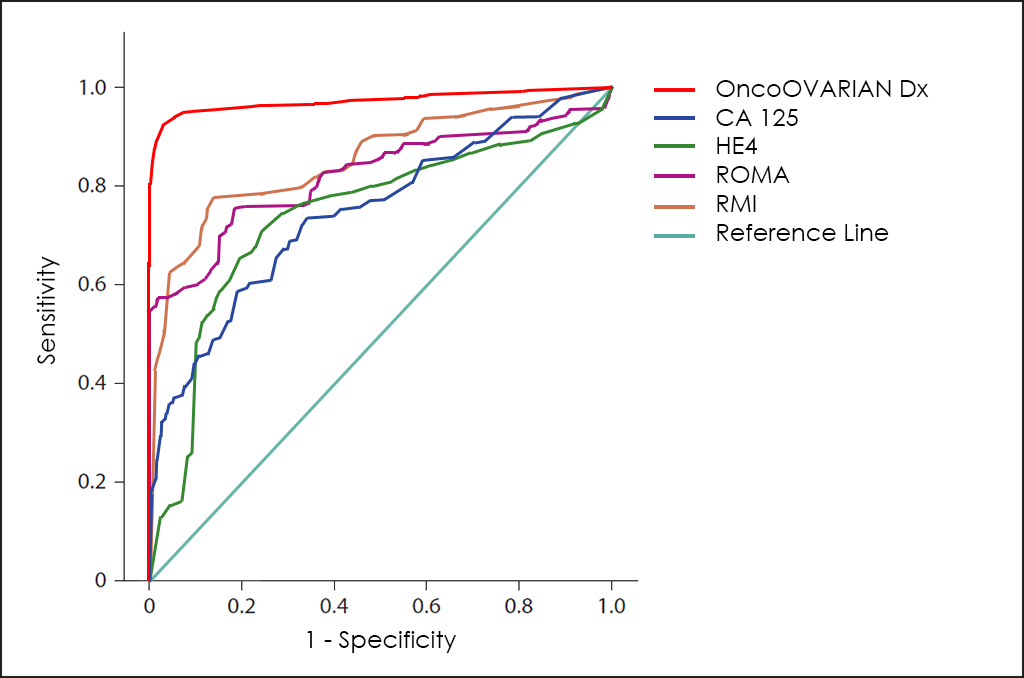
The ROC curve of OncoOVARIAN Dx test ―based on the combined count of AFP, β-hCG, CA 19.9, CA 125, CEA and HE4 Tumor Markers; comorbidities; and other data from 4.520 consecutive patients, then fine-tuned by other research―, throws really interesting diagnostic capabilities: 93.5% Sensitivity and 94.3% Specificity.
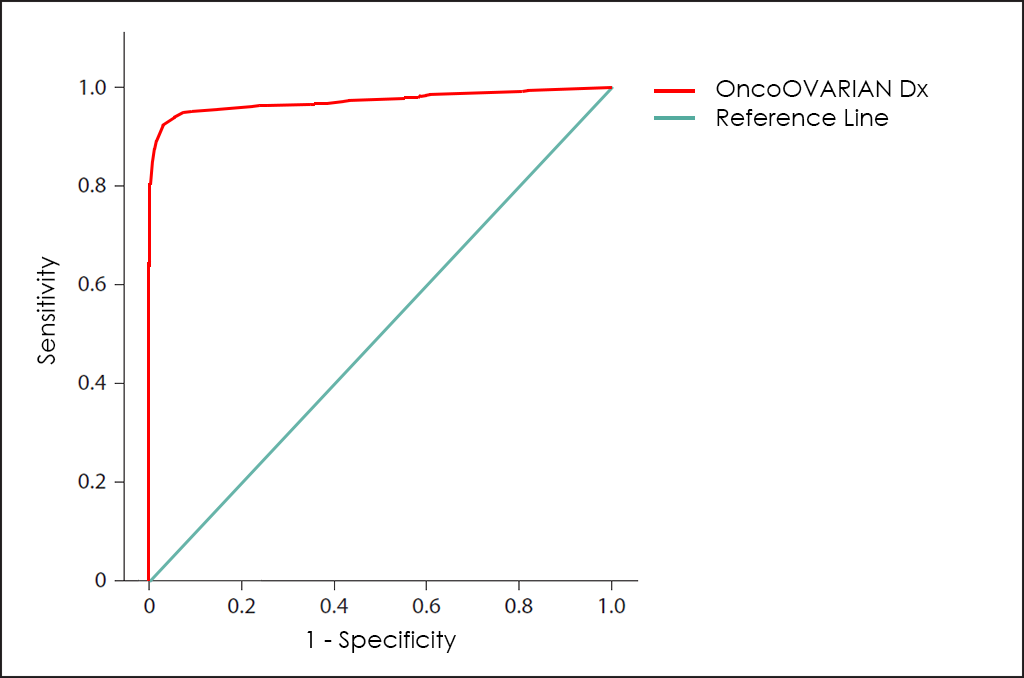
Besides Risk: Histologycal Type
OncoOVARIAN Dx test also allow us to determine with that so high accuracy the Histologycal Type, distinguishing between Epithelial Tumors, Germ Cell Tumors or Sex Cords-Stromal Tumors.
How does it work
As all BIOPROGNOS’ MBDAA tests, OncoOVARIAN Dx test is available online once access is granted through our secure Cloud Platform. As a Cloud solution, it is designed to be used in a Software as a Service (SaaS) basis, that means, no installation, no periodically patch upgrades, low TCO (Total Cost of Ownership) and no maintenance.
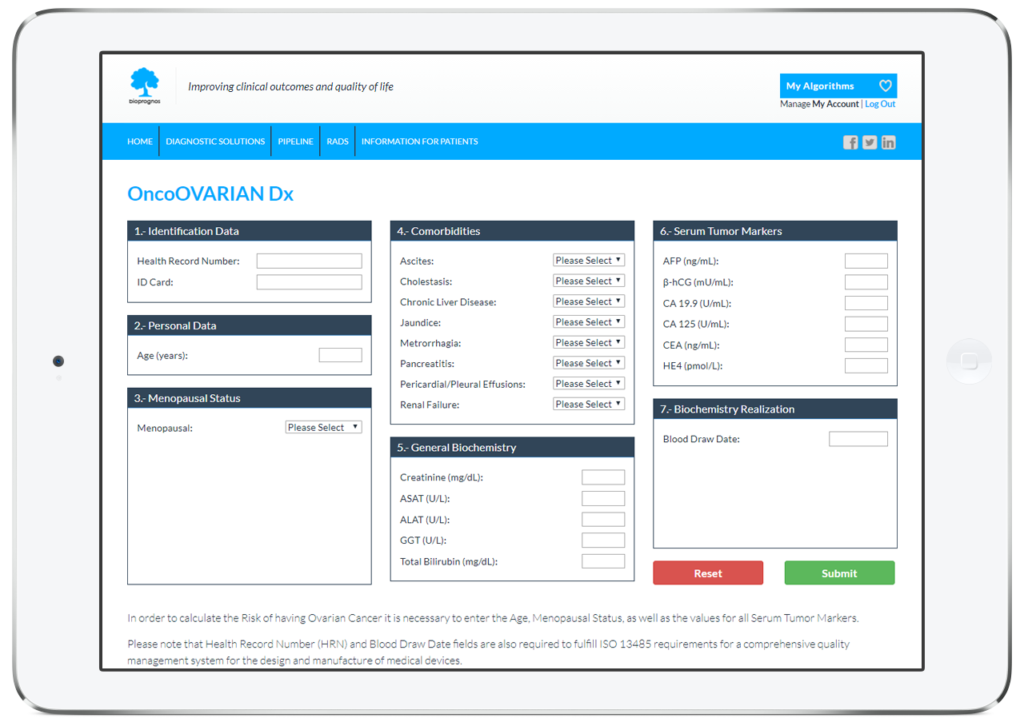
In this way, doctors or lab technicians only should fill the form with values obtained previously from patients (personal data, family background, menopausal status, comorbidities, biochemistry values, CT Scan/US finding, medications and prior procedures information), and click on Submit button in order to obtain the risk score of having Ovarian Cancer.
Order Form
To facilitate work, doctors can download and fill the Order Form for OncoOVARIAN Dx in a quick and an easy way ―with all required data for a best risk calculation already detailed―.
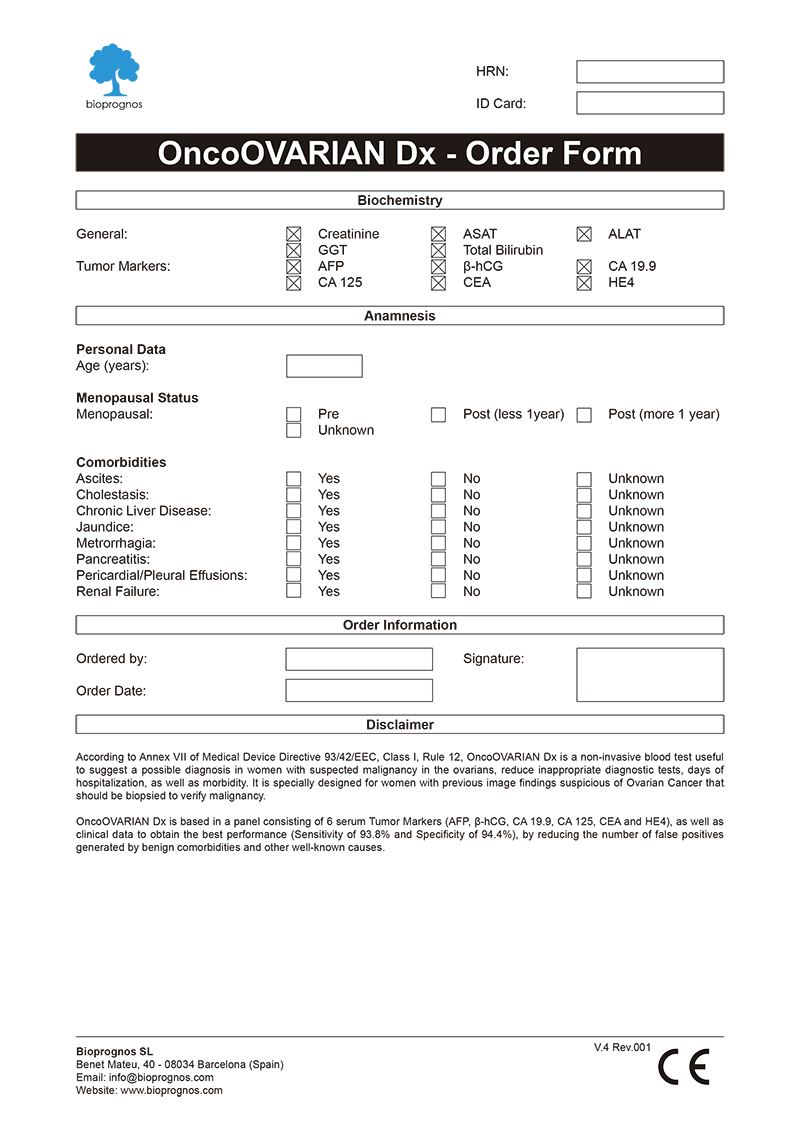
Click here to download the OncoOVARIAN Dx Order Form in PDF format.
Final Report
After doctors entered the patient’s data, OncoOVARIAN Dx test presents the results in a separate screen that can be converted to a PDF document in order to be downloaded or sent by email.
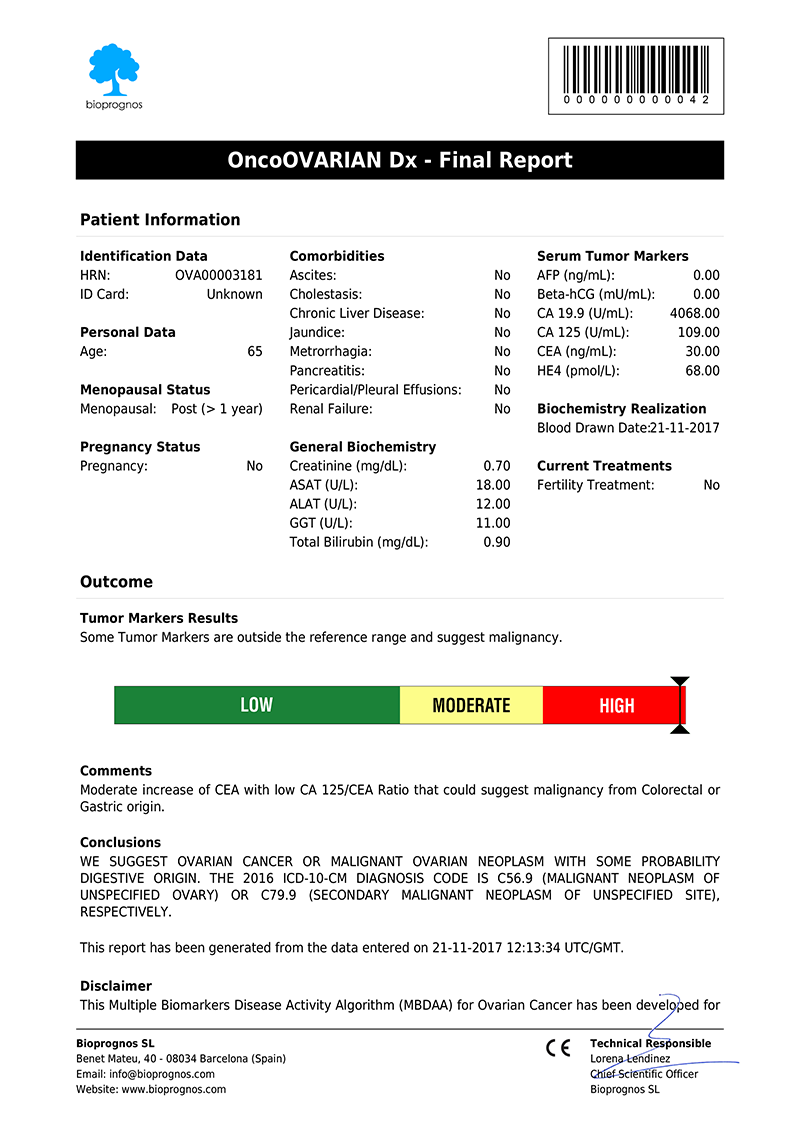

Click here to download the report in PDF format.
The report includes two main sections: Patient Information and Outcome. In the first one, all patient data entered previously is showed as record. The second one includes: Results, indicating whether Tumor Marker levels are within normal range or not; Risk, with a score bar showing the probability of having Ovarian Cancer; Comments, that are created dynamically to help doctors and healthcare professionals to understand ―in an easy way―, how to detect False Positives (FP), such as levels of Tumor Markers that would suspect the presence of Cancer, but when considering other variables together ―Age, Menopausal Status or Comorbidities information―, do not correspond with malignant diseases; and finally, Conclusions, with recommendations suggesting to retest patient in 1 year (for Low Risk), or in 4 weeks (for Moderate Risk, that is, these cases in which Tumor Marker levels are higher than normality but there is not quite clear to be High Risk.
Please note that final report is oriented to healthcare professionals only ―not to patients―, because it was designed as “a tool to help healthcare professionals in Ovarian Cancer diagnostic”, and in this way it is been certified by obtaining the CE DECLARATION OF CONFORMITY (Medical Device Directive 93/42/EEC, Class I, rule 12).
CE Declaration of Conformity
Since April 12th, 2017 (when version 1.0 was released), OncoOVARIAN Dx test has the CE Declaration of Conformity that certifies it has been assessed to meet high safety, health, and environmental protection requirements.

Click here to download the OncoOVARIAN Dx CE DECLARATION OF CONFORMITY in PDF format.
This declaration also certifies that OncoLUNG test can be sold throughout the European Economic Area (EEA) without restrictions.
Besides, there are two main benefits CE marking brings to businesses and consumers within the EEA:
- Businesses know that products bearing the CE marking can be traded in the EEA.
- Consumers enjoy the same level of health, safety, and environmental protection throughout the entire EEA.
Other tests in the market
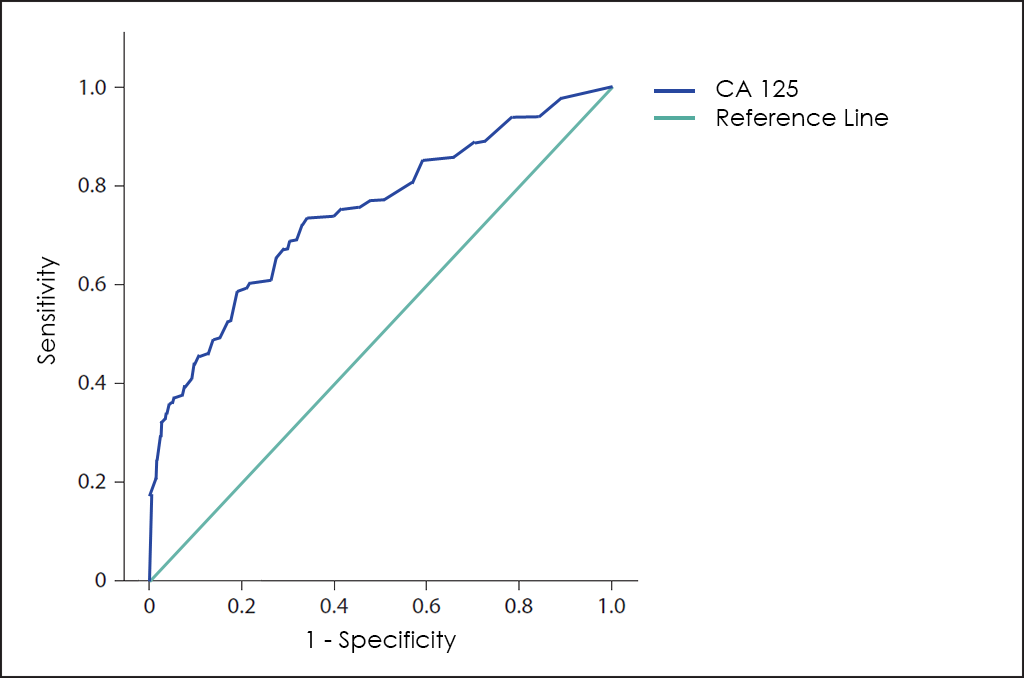
Different methods for early diagnosis have been developed, including the use of the CA 125 Tumor Marker, but the results are not satisfactory, since this marker has a low Sensitivity in the early stages while offering a high proportion of false positives (FP) in premenopausal women.
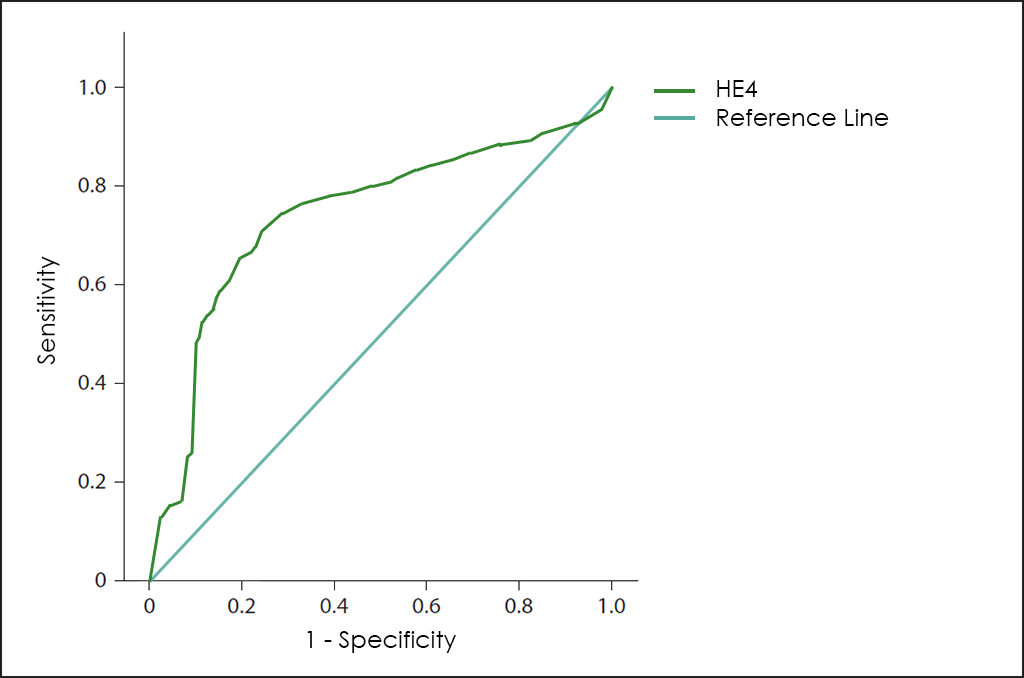
For some years, the valuation levels of HE4 ―a novel Tumor Marker―, offers greater sensitivity for these early stages, and greater specificity.
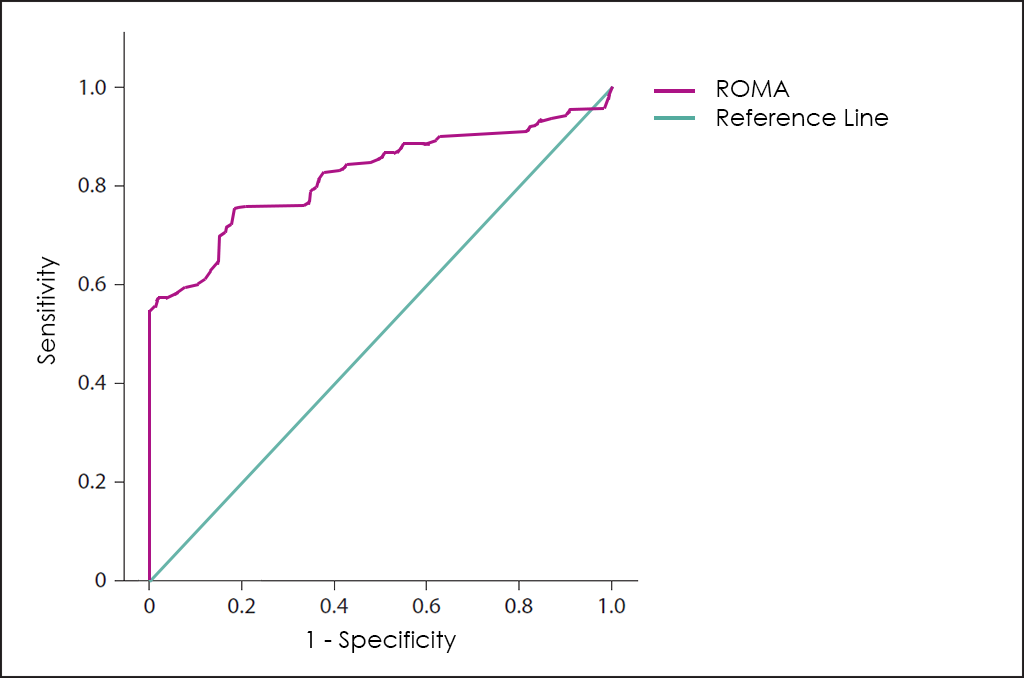
A combination of the values of CA125, HE4 and menopausal status of the patients, the ROMA test (Risk of Ovarian Malignancy Algorithm) ―a 3 variables and low complexity to help physicians in the Ovarian Cancer diagnosis―.
Another different approach sought to improve early diagnosis by combining the CA125 and characteristics of the ovarian mass obtained by Ultrasound, which led to the RMI (Risk of Malignancy Index for Ovarian Cancer) test.
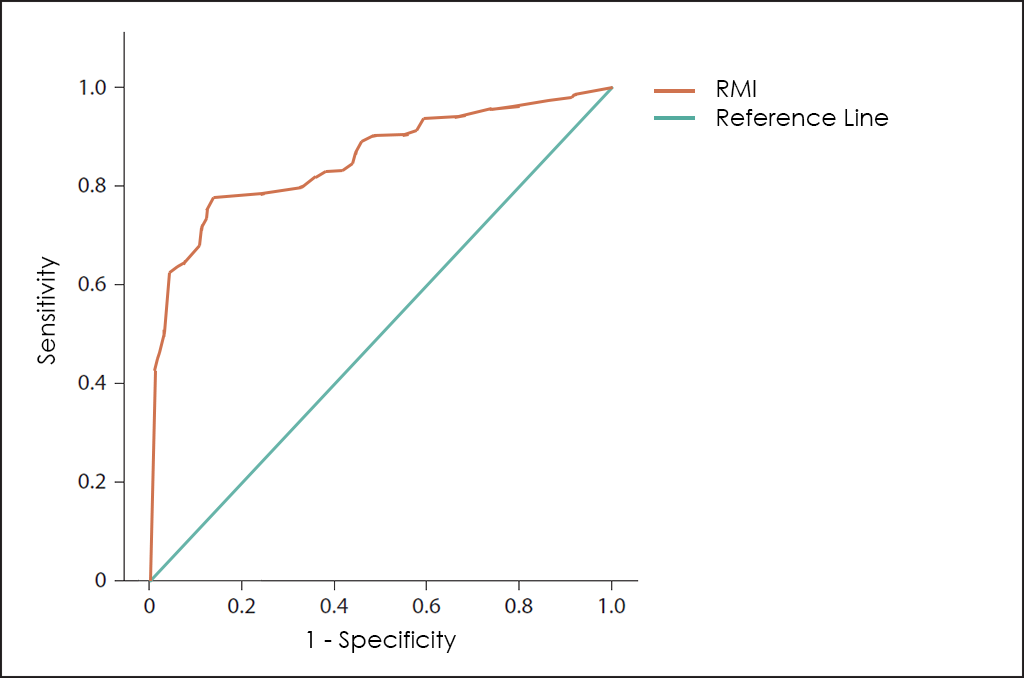
Both test are useful to help in the diagnosis of abdominal masses ―as well as the monitoring of treatment―, but both can be clearly improved because ROMA does not consider age or comorbilities of the patient and RMI does not use the best Tumor Marker to date for the diagnosis of Ovarian Cancer ―the HE4―, besides other variables that we have found as valuable to this disease in OncoOVARIAN Dx.
Superposed ROC curves for the markers CA125, HE4, ROMA, RMI and OncoOVARIAN Dx for comparison are as follows:
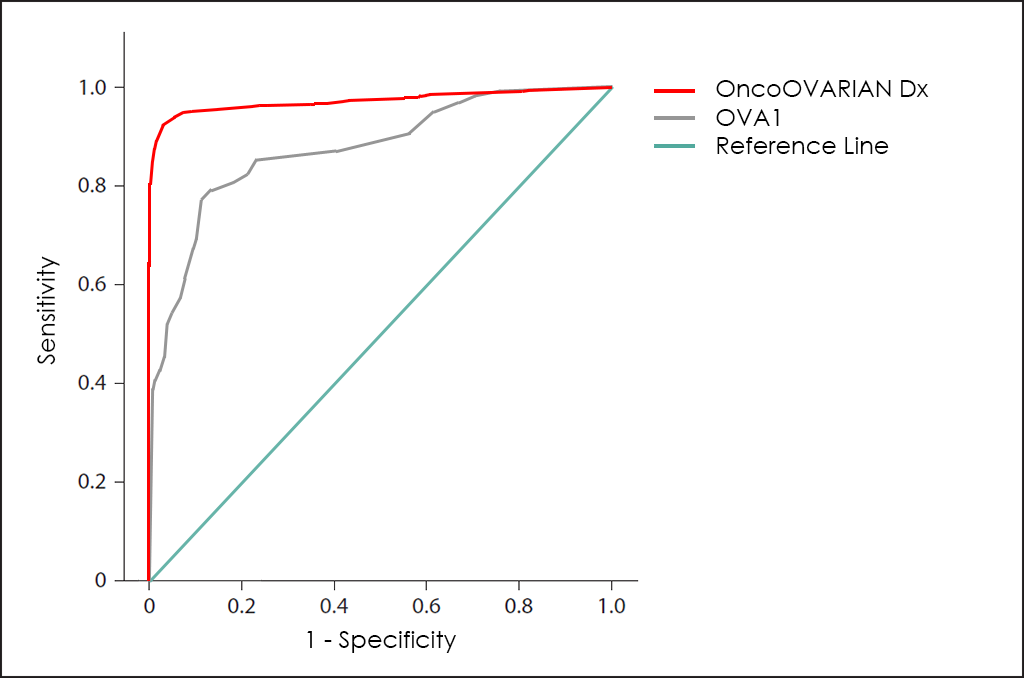
Finally, Vermillion (NasdaqCM: VRML), an American biotech company, have developed the OVA1 test to also help physicians make the right treatment decisions for patients with pelvic masses and avoid unnecessary surgeries.
OVA1 is based in 5 biomarkers found in serum, such as Apolipoprotein A1, β2 Microglobulin, CA 125, Prealbumin and Transferrin, and the global performance is greater than ROMA and RMI, but still poor if compared with our OncoOVARIAN Dx test, as you can see in superposed ROC curves for OVA1 and OncoOVARIAN Dx tests:
Uses and purposes for OncoOVARIAN Dx
OncoOVARIAN Dx test has been developed for:
- Aid in diagnostic assessments for high-risk patients (women older than 40 years with Ovarian or Breast Cancer family history).
- Confirm or discard malignancy from results obtained previously with other tests, such as Computed Tomography (CT) Scan or Ultrasound (US) findings thanks higher Sensitivity and Specificity than imaging procedures.
- Help doctors predict the cancer’s behaviour and response to treatment, as well as a person’s chance of recovery.
- Guide treatment decisions (such as decide whether to add or immunotherapy after surgery and/or radiation therapy), therapy monitoring (doctors may use changes in the presence or amount of one or more Tumor Markers to assess how the cancer is responding to treatment) and predict or monitor for recurrence (looking for changes in the amount of a Tumor Marker may be part of their follow-up care plan and may help detect a recurrence sooner than other methods).
The Science Behind OncoOVARIAN Dx
Based on Publications
- Molina, R., Auge J. M., Escudero J. M., Filella X., Foj, L., Torné A., Lejarcegui J., Pahisa J. “HE4 a novel tumour marker for ovarian cancer: comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases.” Tumour Biol, 2011. 32(6): p. 1087-95. PMCID: PMC3195682
- Santotoribio J.D., Garcia-de la Torre A., Cañavate-Solano C., Arce-Matute F., Sanchez-del Pino M.J., Perez-Ramos S. “Cancer antigens 19.9 and 125 as tumor markers in patients with mucinous ovarian tumors.” EJGO European Journal of Gynaecological Oncology. PMID: 27048105
- Shaikh N. A., Memon F., Samo R. P. “Tumor markers; efficacy of CA-125, CEA, AFP and Beta-HCG. An institutional based descriptive & prospective study in diagnosis of ovarian malignancy.” Professional Med J 2014;21(4):621-627. PDF
- Sørensen S. S., Mosgaard B. J. “Combination of CA 125 and CEA can improve ovarian cancer diagnosis.” DANISH MEDICAL BULLETIN. Dan Med Bul 2011;58(11):A4331. November 2011. PDF
- Molina R., Filella X., Trapé J., Augé J. M., Barco A., Cañizares F., Colomer A., Fernandez A., Gaspar M. J., Martinez-Peinado A., Pérez L., Sánchez M., Escudero J. M. (2013). “Principales causas de falsos positivos en los resultados de marcadores tumorales en suero.” Sociedad Española de Bioquímica Clínica y Patología Molecular. Comisión de Marcadores Biológicos del Cáncer. PDF
Related Publications
- Abbas, A. M., et al., A new scoring model for characterization of adnexal masses based on two-dimensional gray-scale and colour Doppler sonographic features. Facts Views Vis Obgyn, 2014. 6(2): p. 68-74. PMCID: PMC4086018
- Akturk, E., et al., Comparison of four malignancy risk indices in the detection of malignant ovarian masses. J Gynecol Oncol, 2011. 22(3): p. 177-82. PMCID: PMC3188716
- Anton, C., et al., A comparison of CA125, HE4, risk ovarian malignancy algorithm (ROMA), and risk malignancy index (RMI) for the classification of ovarian masses. Clinics (Sao Paulo), 2012. 67(5): p. 437-41. PMCID: PMC3351260
- Antovska, V. and M. Trajanova, An original risk of ovarian malignancy index and its predictive value in evaluating the nature of ovarian tumour. Southern African Journal of Gynaecological Oncology, 2015. 7(2): p. 52-59. PDF
- Barber, E. L., et al., A preoperative personalized risk assessment calculator for elderly ovarian cancer patients undergoing primary cytoreductive surgery. Gynecol Oncol, 2015. 139(3): p. 401-6. DOI: 10.1016/j.ygyno.2015.09.080
- Bristow, R. E., et al., A model for predicting surgical outcome in patients with advanced ovarian carcinoma using computed tomography. Cancer, 2000. 89(7): p. 1532-40. PMID: 11013368
- Duffy, M. J. (2010). “Clinical Utility of Tumor Markers: What the Guidelines Recommend.” Journal of Laboratory Diagnostics 46(3): 281-291. PDF
- Duffy, M. J. and P. McGing (2010). Guidelines for the use of tumour markers. PDF
- Eagle, K. and J.A. Ledermann, Tumor Markers in Ovarian Malignancies. Oncologist, 1997. 2(5): p. 324-329. PDF
- G, S., et al., Guidelines for Use of Biomarkers in Gynecological Cancer: 2012, in Gynecological Cancer. 2012, European Group of Tumor Markers (EGTM). PDF
- Hasholzner, U., et al., Significance of the tumour markers CA 125 II, CA 72-4, CASA and CYFRA 21-1 in ovarian carcinoma. Anticancer Res, 1994. 14(6B): p. 2743-6. PubMed: 7532929
- JW, P., et al., Four risk of malignancy indices in evaluation of pelvic masses. Korean J Obstet Gynecol., 2012. 55(9): p. 636-643. PDF
- Kaijser, J., et al., Improving strategies for diagnosing ovarian cancer: a summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet Gynecol, 2013. 41(1): p. 9-20. DOI: 10.1002/uog.12323
- Karlsen, M. A., et al., A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer – An international multicenter study in women with an ovarian mass. Gynecol Oncol, 2015. 138(3): p. 640-6. DOI: 10.1016/j.ygyno.2015.06.021
- Kim, Y. W., et al., Multiplexed bead-based immunoassay of four serum biomarkers for diagnosis of ovarian cancer. Oncol Rep, 2012. 28(2): p. 585-91. DOI: 10.1371/journal.pone.0044960
- Lawicki, S., et al., The plasma concentration of VEGF, HE4 and CA125 as a new biomarkers panel in different stages and sub-types of epithelial ovarian tumors. J Ovarian Res, 2013. 6(1): p. 45. PMCID: PMC3706238
- Li, A., New biomarkers for ovarian cancer: OVA1 and ROMA in diagnosis. 2012. DOC
- Mayeux, R. (2004). “Biomarkers: potential uses and limitations.” NeuroRx 1(2): 182-188. DOI: 10.1602/neurorx.1.2.182
- Medicine, T. A. f. c. B. L. (2013). RECOMMENDATIONS AS A RESULT OF THE ACB NATIONAL AUDIT ON TUMOUR MARKER SERVICE PROVISION. PDF
- Moore, R. G., et al., A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol, 2009. 112(1): p. 40-6. PMCID: PMC3594094
- Moore, R. G., et al., Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol, 2010. 203(3): p. 228 e1-6. DOI: 10.1016/j.ajog.2010.03.043
- Novakovic, S. (2004). “Tumor markers in clinical oncology.” Radiology and Oncology 38(2). PDF
- P, I. and P. N, Evaluation of Four Risk of Malignancy Indices (RMI) in the Preoperative Diagnosis of Ovarian Malignancy at Rajavithi Hospital. Thai Journal of Obstetrics and Gynaecology, 2013. 21: p. 163-175. PDF
- Perez, E. O. and M. I. Aceituno Azaustre (2014). “Utilidad clínica de los marcadores tumorales.” Revista Médica de JAÉN(4): 2-12. PDF
- Perkins, G. L., E. D. Slater, G. K. Sanders and J. G. Prichard (2003). “Serum Tumor Markers.” American Family Physician 68(6): 1075 – 1082. PDF
- Pinsky, P. F., et al., Potential effect of the Risk of Ovarian Cancer Algorithm (ROCA) on the mortality outcome of the Prostate, Lung, Colorectal and Ovarian (PLCO) trial. Int J Cancer, 2013. 132(9): p. 2127-33. DOI: 10.1002/ijc.27909
- Pitta Dda, R., et al., Symptoms, CA125 and HE4 for the preoperative prediction of ovarian malignancy in Brazilian women with ovarian masses. BMC Cancer, 2013. 13: p. 423. DOI: 10.1186/1471-2407-13-423
- Rein, B. J., et al., Potential markers for detection and monitoring of ovarian cancer. J Oncol, 2011. 2011: p. 475983. DOI: 10.1155/2011/475983
- Sayasneh, A., et al., Evaluating the risk of ovarian cancer before surgery using the ADNEX model: a multicentre external validation study. Br J Cancer, 2016. 115(5): p. 542-8. DOC
- Schmidt, C., CA-125: a biomarker put to the test. J Natl Cancer Inst, 2011. 103(17): p. 1290-1. DOI: 10.1093/jnci/djr344
- Sharma, S. (2009). “Tumor markers in clinical practice: General principles and guidelines.” Indian J Med Paediatr Oncol 30(1): 1-8. PMCID: PMC2902207
- Skates, S. J., Ovarian cancer screening: development of the Risk of Ovarian Cancer Algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer, 2012. 22 Suppl 1: p. S24-6. PMCID: PMC3572791
- Sladkevicius, P. and L. Valentin, Intra- and interobserver agreement when describing adnexal masses using the International Ovarian Tumor Analysis terms and definitions: a study on three-dimensional ultrasound volumes. Ultrasound Obstet Gynecol, 2013. 41(3): p. 318-27. DOI: 10.1002/uog.12289
- Soletormos, G., et al., Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int J Gynecol Cancer, 2016. 26(1): p. 43-51. DOI: 10.1097/IGC.0000000000000586
- Sturgeon, C. (2002). “Practice guidelines for tumor marker use in the clinic.” Clin Chem 48(8): 1151-1159. PMID: 12142367
- Sturgeon, C. M., B. R. Hoffman, D. W. Chan, S. L. Ch’ng, E. Hammond, D. F. Hayes, L. A. Liotta, E. F. Petricoin, M. Schmitt, O. J. Semmes, G. Soletormos, E. van der Merwe, E. P. Diamandis and B. National Academy of Clinical (2008). “National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines for use of tumor markers in clinical practice: quality requirements.” Clin Chem 54(8). DOI: 10.1373/clinchem.2007.094144
- Timmerman, D., et al., Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: a temporal and external validation study by the IOTA group. Ultrasound Obstet Gynecol, 2010. 36(2): p. 226-34. DOI: 10.1002/uog.7636
- Trape, J., R. Molina, F. Sant, J. Montesinos, A. Arnau, J. Franquesa, R. Blavia, E. Martin, E. Marquilles, D. Perich, C. Perez, J. M. Roca, M. Domenech, J. Lopez and J. M. Badal (2012). “Diagnostic accuracy of tumour markers in serous effusions: a validation study.” Tumour Biol 33(5): 1661-1668. DOI: 10.1007/s13277-012-0422-3
- Trapé Pujol, J. and R. Molina Porto (2006). “Aspectos generales de los marcadores tumorales.” JANO 1620: 45-48. PDF
- Van Calster, B., et al., Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn, 2015. 7(1): p. 32-41. PMCID: PMC4402441
- Yamamoto, Y., et al., Comparison of 4 Risk-of-Malignancy Indexes in the Preoperative Evaluation of Patients With Pelvic Masses: A Prospective Study. Clinical Ovarian & Other Gynecologic Cancer 2014. 7(1): p. 8-12. DOI: 10.1016/j.cogc.2014.11.001
- Yoruk, P., et al., Comparison of the risk of malignancy index and self-constructed logistic regression models in preoperative evaluation of adnexal masses. J Ultrasound Med, 2008. 27(10): p. 1469-77. PDF
- Yoshida, A.,et al., Comparing the Copenhagen Index (CPH-I) and Risk of Ovarian Malignancy Algorithm (ROMA): Two equivalent ways to differentiate malignant from benign ovarian tumors before surgery? Gynecol Oncol, 2016. 140(3): p. 481-5. DOI: 10.1016/j.ygyno.2016.01.023

