OncoBREAST Dx – Breast Cancer Diagnosis Test
Overview
OncoBREAST Dx is an innovative, non-invasive, accurate, and cost-effective already validated solution to help in Breast Cancer diagnosis as well as confirmatory diagnostic ―as an adjunct to suspicious image procedures findings, in order to reduce the number of unnecessary tissue biopsies that patients have to undergo―. It also has potentially uses for screening, prognosis and recurrence monitoring.
OncoBREAST Dx is based on a score calculation that it is obtained from several Biomarkers of the patient (mainly Tumor Markers but also patient’s clinical information).

Click here to download the brochure in PDF format.
Tumor Markers
Tumor Markers are parameters released by tumor cells, which enter the bloodstream or other biological fluids and are useful for the diagnosis, prognosis and treatment monitoring.
Most Tumor Markers are not specific to any type of cancer and the differences between benign and malignant diseases are quantitative (for example, patients with epithelial tumors tend to have significantly higher levels of these Tumor Markers than patients without malignancy).
There are now more than 20 well known parameters that are widely regarded as Tumor Markers such as PSA ―related to Prostate Cancer―, CA 15.3 ―related to Breast Cancer―, CA 125 and HE4 ―both related to Ovarian Cancer―, CEA and CA 19.9 ―both related to different gastrointestinal cancers (Colorectal, Gastric and Pancreatic Cancer)―, or NSE and ProGRP ―both related to in Lung Cancer―.
However, there are a variety of factors that can affect the accuracy of Tumor Markers by increasing its levels without malignancy presence. The main reason are benign diseases, among others, such as technical interferences.
In this sense, the Spanish Society of Clinical Biochemistry and Molecular Pathology, Cancer Biomarker Commission established the Barcelona Criteria, 4 criteria that help to correctly distinguish and value Tumor Markers results and reduce False Positives (FP):
- Tumor Markers Serum concentrations.
- Discard benign pathology by the exclusion of main source False Positive results.
- Follow-up if Tumor Markers moderate results (Grey Zone/Undetermined).
- Technical interference.
Statistical measurements in diagnostic tests
Unfortunately, the use of Tumor Markers in routine presents also other problems such as low Sensitivity in early stages, or nonexistence of any specific Tumor Marker for each malignant tumor. However, the combination of 2 or more Tumor Markers has a better outcome, especially in advanced stages.
In this regard, the combination of several Tumor Markers ―as well as the inclusion of patient history information in the equations―, using complex algorithms with multiple variables, results in higher Sensitivity and Specificity: that is what we have christened Multi-Biomarker Disease Activity Algorithm (MBDAA).
The Sensitivity of a diagnostic test is the percentage of actual positives that are correctly identified, and Specificity is the proportion of true negatives that are correctly classified. Both variables are closely linked together and give an idea of the accuracy of a test.
A test that correctly identifies all true positive as positive, but has many false negatives would have a Sensitivity of 100%, but low Specificity. For example, Sensitivity measures the number of cancerous tumors that are correctly identified as cancerous, whereas Specificity measures how many benign tumors are correctly identified as benign. A high Sensitivity means fewer cancers diagnosed as benign and high Specificity means fewer benign tumors diagnosed as cancerous.
Besides, the positive predictive value (PPV) is the number of true positives correctly identified on total real positive. A test with many false positives will have a low VPP. Moreover, the negative predictive value (NPV) is the number of true negatives correctly identified on the total actual negative. A high NPV value means that very few true positives were incorrectly identified as negative.
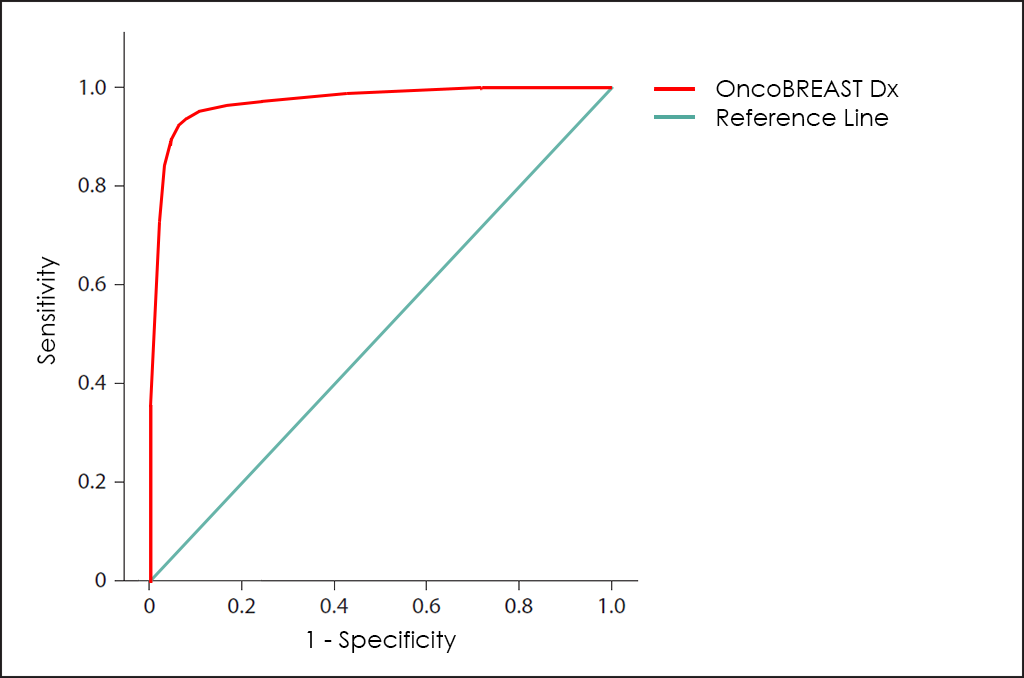
All these different values can be plotted together in a graphic that it is known as Receiving Operator Curve (ROC), where better results are displayed with curves that tends to come near to the upper left corner of the image (where 100% Sensitivity and 100% Specificity are reached).
Receiving Operator Curves (ROC)
The ROC curve of OncoBREAST Dx test ―based on the combined count of CA 15.3, CEA, EGFR, NSE, NGAL and 8-OHdG Tumor Markers; comorbidities and other data patients, then fine-tuned by other research―, throws really interesting diagnostic capabilities: 91.7% Sensitivity and 93.6% Specificity.
How does it work
As all BIOPROGNOS’ MBDAA tests, OncoBREAST Dx test is available online once access is granted through our secure Cloud Platform. As a Cloud solution, it is designed to be used in a Software as a Service (SaaS) basis, that means, no installation, no periodically patch upgrades, low TCO (Total Cost of Ownership) and no maintenance.
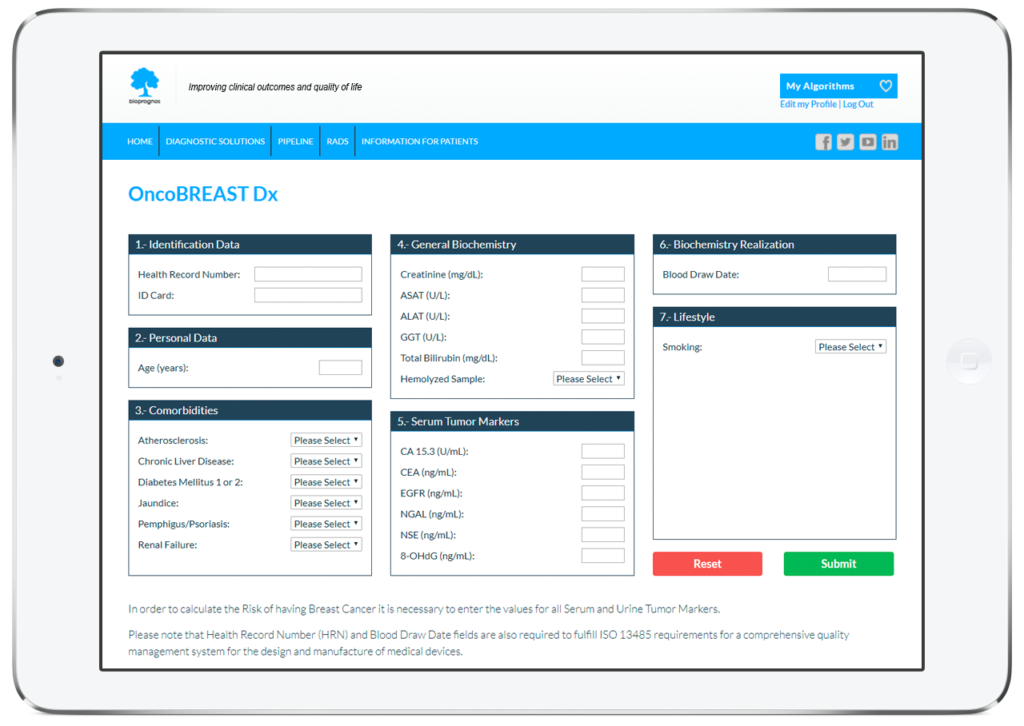
In this way, doctors or lab technicians only should fill the form with values obtained previously from patients (personal data, comorbidities, biochemistry values, mammography finding and lifestyle information), and click on Submit button in order to obtain the risk score of having Breast Cancer.
Order Form
To facilitate work, doctors can download and fill the Order Form for OncoBREAST Dx in a quick and an easy way ―with all required data for a best risk calculation already detailed―.
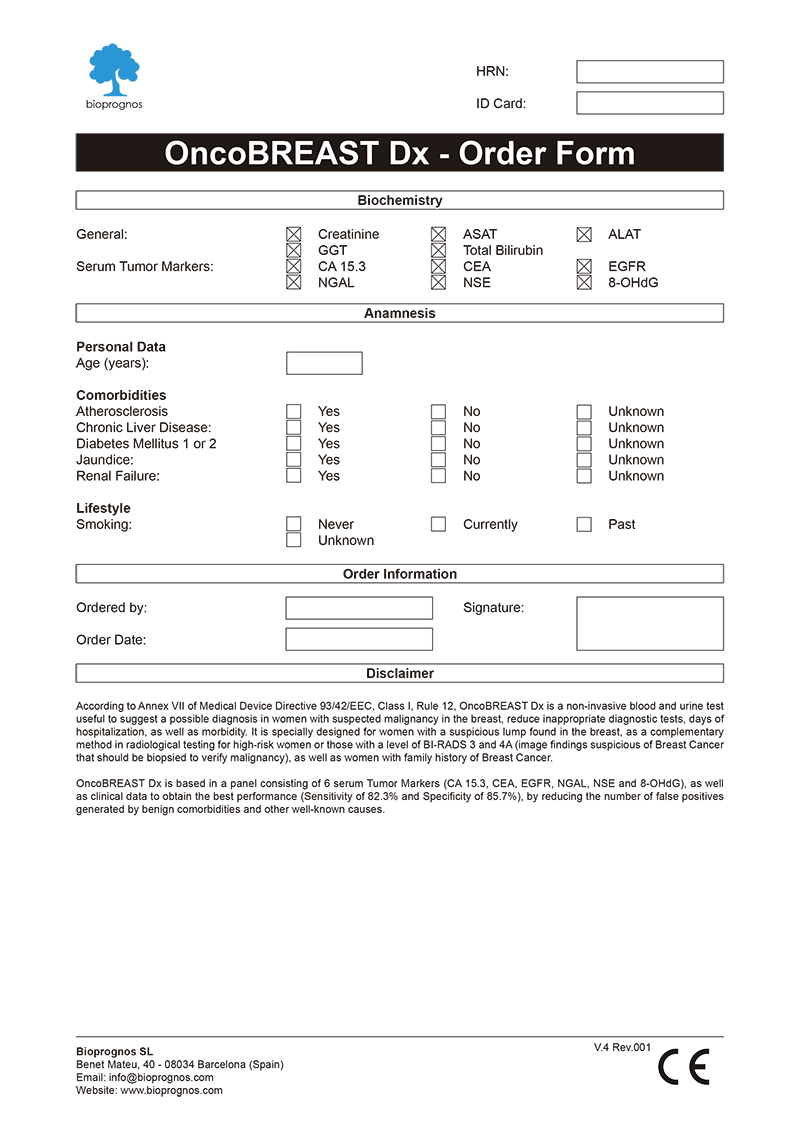
Click here to download the OncoBREAST Dx Order Form in PDF format.
Final Report
After doctors entered the patient’s data, OncoBREAST Dx test presents the results in a separate screen that can be converted to a PDF document in order to be downloaded or sent by email.
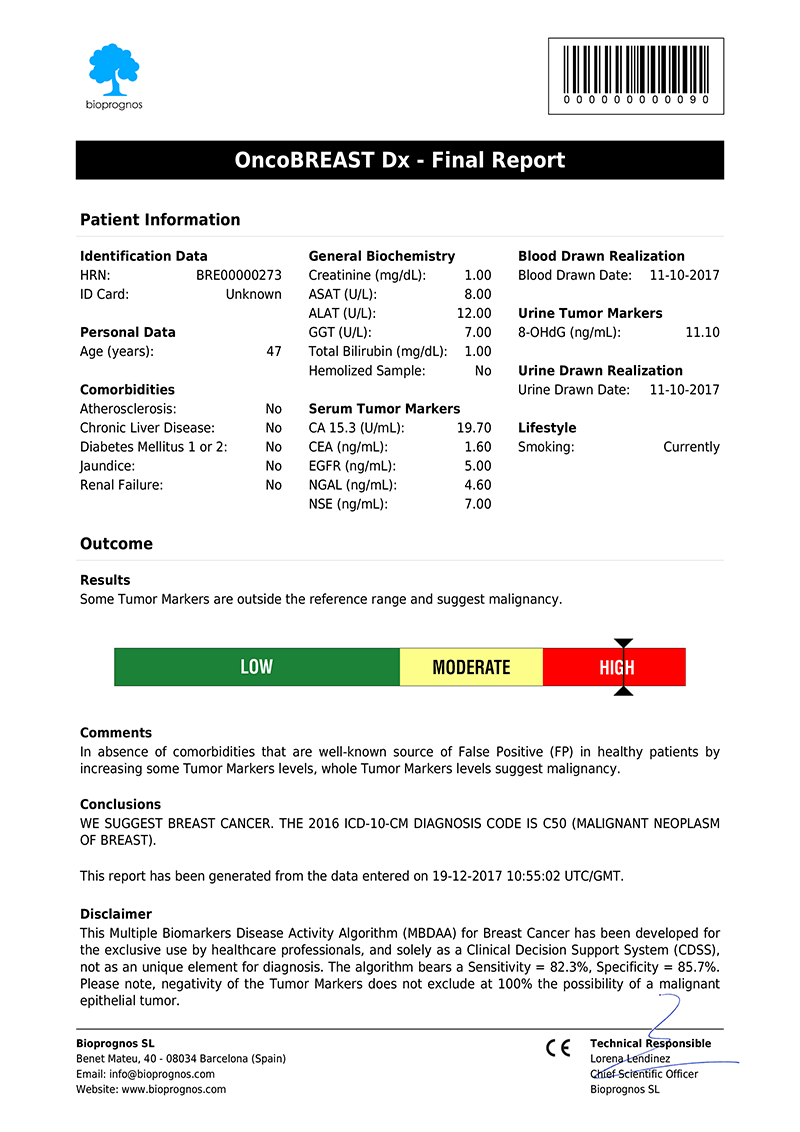
Click here to download the report in PDF format.
The report includes two main sections: Patient Information and Outcome. In the first one, all patient data entered previously is showed as record. The second one includes: Results, indicating whether Tumor Marker levels are within normal range or not; Risk, with a score bar showing the probability of having Breast Cancer; Comments, that are created dynamically to help doctors and healthcare professionals to understand ―in an easy way―, how to detect False Positives (FP), such as levels of Tumor Markers that would suspect the presence of Cancer, but when considering other variables together ―Comorbidities or Smoking Habits―, do not correspond with malignant diseases; and finally, Conclusions, with recommendations suggesting to retest patient in 1 year (for Low Risk), or in 4 weeks (for Moderate Risk, that is, these cases in which Tumor Marker levels are higher than normality but there is not quite clear to be High Risk.
Please note that final report is oriented to healthcare professionals only ―not to patients―, because it was designed as “a tool to help healthcare professionals in Breast Cancer diagnostic”, and also certified by obtaining the CE DECLARATION OF CONFORMITY (Medical Device Directive 93/42/EEC, Class I, rule 12).
CE Declaration of Conformity
OncoBREAST Dx test has the CE Declaration of Conformity that certifies it has been assessed to meet high safety, health, and environmental protection requirements.

Click here to download the OncoBREAST Dx CE DECLARATION OF CONFORMITY in PDF format.
This declaration also certifies that OncoBREAST Dx test can be sold throughout the European Economic Area (EEA) without restrictions.
Besides, there are two main benefits CE marking brings to businesses and consumers within the EEA:
- Businesses know that products bearing the CE marking can be traded in the EEA.
- Consumers enjoy the same level of health, safety, and environmental protection throughout the entire EEA.
Uses and purposes for OncoBREAST Dx
OncoBREAST Dx test has been developed for:
- Aid in diagnostic assessments for high-risk patients (both women with family history of Breast Cancer older than 40 years ―for which risk is doubled if one first-degree relatives have been diagnosed, or risk is 5 times higher than average if two first-degree relatives have been diagnosed―; women having an abnormal gene, such as the BRCA1 or BRCA2 gene as well as abnormal CHEK2 gene).
- Women with a suspicious lump found in the breast.
- Confirm or discard malignancy from results obtained previously with other tests, such as Mammography or Computed Tomography (CT) Scan findings thanks higher Sensitivity and Specificity than imaging procedures, as a complementary method for those with a level of BI-RADS 3 and 4A (image findings suspicious of Breast Cancer that should be biopsied to verify malignancy).
- Help doctors predict the cancer’s behaviour and response to treatment, as well as a person’s chance of recovery.
- Guide treatment decisions (such as decide whether to add or immunotherapy after surgery and/or radiation therapy), therapy monitoring (doctors may use changes in the presence or amount of one or more Tumor Markers to assess how the cancer is responding to treatment) and predict or monitor for recurrence (looking for changes in the amount of a Tumor Marker may be part of their follow-up care plan and may help detect a recurrence sooner than other methods).
The Science Behind OncoBREAST Dx
Based on Publications
- Bayo J., Castaño M. A., Rivera F. and Navarro F. (2017). “Analysis of blood markers for early breast cancer diagnosis”. Clin Transl Oncol. Springer. DOI: 10.1007/s12094-017-1731-1
- Molina R., Filella X., Trapé J., Augé J. M., Barco A., Cañizares F., Colomer A., Fernandez A., Gaspar M. J., Martinez-Peinado A., Pérez L., Sánchez M., Escudero J. M. (2013). “Principales causas de falsos positivos en los resultados de marcadores tumorales en suero”. Sociedad Española de Bioquímica Clínica y Patología Molecular. Comisión de Marcadores Biológicos del Cáncer. PDF
Related Publications
- Asgeirsson K. S., Agrawal A., Allen C., Hitch A., Ellis I. O., Chapman C., Cheung K. L. and Robertson J. F. “Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients”. Breast Cancer Res. 2007;9(6):R75. DOI: 10.1186/bcr1788
- Duffy M.J., Harbeck N., Nap M., Molina R., Nicolini A., Senkus E. and Cardoso F. “Clinical use of biomarkers in breast cancer – Updated Guidelines from the European Group on Tumor Markers (EGTM)”. M.J. Duffy et al. / European Journal of Cancer 75 (2017) 284e298 DOI: 10.1016/j.ejca.2017.01.017
- Kees A. Yedema K. A., Kenemans P., Wobbes T., Van Kamp G. J., De Bruijns H. W., Thomas C. M., Massuger L. F., Schijf C. P., Bon G. G., Vermorken J. B., Voorhorst F. and Hilgers J. (1991). “Carcinoma-Associated Mucin Serum Markers CA M26 and CA M29: Efficacy in Detecting and Monitoring Patients with Cancer of fhe Breast, Colon, Ovary, Endometrium and Cervix”. Int. J . Cancer: 47, 170-179 (1991). Wiley-Liss, Inc. PMID: 1988362
- Ławicki S., Głażewska E. K., Sobolewska M., Będkowska G. E. and Szmitkowski M. (2016). “Plasma Levels and Diagnostic Utility of Macrophage Colony-Stimulating Factor, Matrix Metalloproteinase-9, and Tissue Inhibitor of Metalloproteinases-1 as New Biomarkers of Breast Cancer”. Ann Lab Med. 2016 May;36(3):223229. English. The Korean Society for Laboratory Medicine. DOI: 10.3343/alm.2016.36.3.223
- Ławicki S., Zajkowska M., Głażewska E. K., Szmitkowski M. (2016). “Plasma levels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer”. OncoTargets and Therapy. Dovepress. DOI: 10.2147/OTT.S99959
- Lazarus E., Mainiero M. B., Schepps B., Koelliker S. L. and Livingston L. S. (2006). “BI-RADS Lexicon for US and Mammography: Interobserver Variability and Positive Predictive Value”. Radiology. 2006 May; 239(2):385-91. Epub 2006 Mar 28. DOI: 10.1148/radiol.2392042127
- Martensson J, Bell M, Xu S, Bottai M, Ravn B, Venge P, et al. “Association of plasma neutrophil gelatinase-associated lipocalin (NGAL) with sepsis and acute kidney dysfunction”. Biomarkers. 2013;18:349–56. DOI: 10.3109/1354750X.2013.787460
- Martoni A.,Zamagni C., Bellanova B., Zanichelli L., (1995). “CEA, MCA, CA 15.3 and CA 549 and their combinations in Expressing and Monitoring Metastatic Breast Cancer: a Prospective Comparative Study”. European Journal of Cancer Vol. 31A, No. 10 pp. 1615-1621, 1995. Elsevier Science Ltd. PMID: 7488411
- Mendoza H., Cisneros L., Martin-Ramos J. and Arango J. (2009). “BI-RADS 3. ¿Realmente son hallazgos benignos? Variabilidad interobservador”. Anales de Radiología México 2009;2:173-176. Artículos originales. PDF
- Molina R., Augé J. M., Escudero J. M., Filella X., Zanon G., Pahisa J., Farrus B., Muñoz M., Velasco M. (2010). “Evaluation of tumor markers (HER-2/neu oncoprotein, CEA, and CA 15.3) in patients with locoregional breast cancer: prognostic value”. Tumor Biol. (2010) 31:171–180. DOI 10.1007/s13277-010-0025-9. DOI: 10.1007/s13277-010-0025-9
- Molina R., Augé J. M., Farrus B., Zanon G., Pahisa J., Muñoz M., Torné A., Filella X., Escudero J. M., Fernández P., Velasco M. (2010). “Prospective Evaluation of Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 15.3 (CA 15.3) in Patients with Primary Locoregional Breast Cancer”. Clinical Chemistry 56:7, 1148–1157 (2010), Cancer Diagnostics. DOI: 10.1373/clinchem.2009.135566
- Molina R., Barak V., van Dalen A., Duffy M. J., Einarsson R., Gion M., Goike H., Lamerz R., Nap M., Sölétormos G. and Stieber P. “Tumor Markers in Breast Cancer – European Group on Tumor Markers (EGTM)”. Tumor Biol 2005 ;26:281–293 DOI: 10.1159/000089260
- Pande D, Negi R, Karki K, Khanna S, Khanna RS, Khanna HD. “Oxidative damage markers as possible discriminatory biomarkers in breast carcinoma”. Transl Res. 2012;160:411–8. DOI: 10.1089/jwh.2016.5973
- Sedgwick E. L., Ebuoma L., Hamame A., Phalak K., Ruiz-Flores L., Ortiz-Perez T. and Sepulveda K. A. (2015). “BI-RADS update for breast cancer caregivers”. Breast Cancer Res Treat (2015) 150:243–254. 3. DOI 10.1007/s10549-015-3332-4
- Tas F, Bilgin E, Karabulut S, Duranyildiz D. “Clinical significance of serum epidermal growth factor receptor (EGFR) levels in patients with breast cancer”. Cytokine. 2015;71:66–70. DOI: 10.1016/j.cyto.2014.09.001
- Valavanidis A, Vlachogianni T, Fiotakis C. “8-hydroxy-2-deoxyguanosine(8-OHdG): a critical biomarker of oxidative stress and carcinogenesis”. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. DOI: 10.1080/10590500902885684
- Wenners AS, Mehta K, Loibl S, Park H, Mueller B, Arnold N, et al. “Neutrophil gelatinase associated lipocalin (NGAL) predicts response to neoadjuvant chemotherapy and clinical outcome in primary human breast cancer”. PLoS One. 2012;7:e45826. DOI: 10.1371/journal.pone.0045826

